Jun 11, 2025 (Business Insider) – Healthcare technology management, or HTM, is a growing field with promising career opportunities — but it’s one that remains under the radar. It ranked 22nd in the US News & World Report’s list of the 100 best jobs of 2025. And according to a report from the US Bureau of Labor Statistics, jobs in […]
Blog

BRUSSELS, July 22 (Reuters) – The European Commission approved on Tuesday up to 403 million euros ($471 million) in public funding for 10 mostly small and medium-sized companies in a bid to support innovation in medical devices. The funding is expected to unlock an additional 826 million euros ($966 million) in private investments to the […]
DUBLIN, Oct. 9, 2024 /Med-Tech News/ — Medtronic plc, a global leader in healthcare technology, has announced the launch of the Avalus Ultra Bioprosthesis valve in Western Europe during the European Association for Cardio-Thoracic Surgery (EACTS) annual congress in Lisbon. The Avalus Ultra valve, engineered for ease of use at implant and lifetime patient management, is Medtronic’s most […]
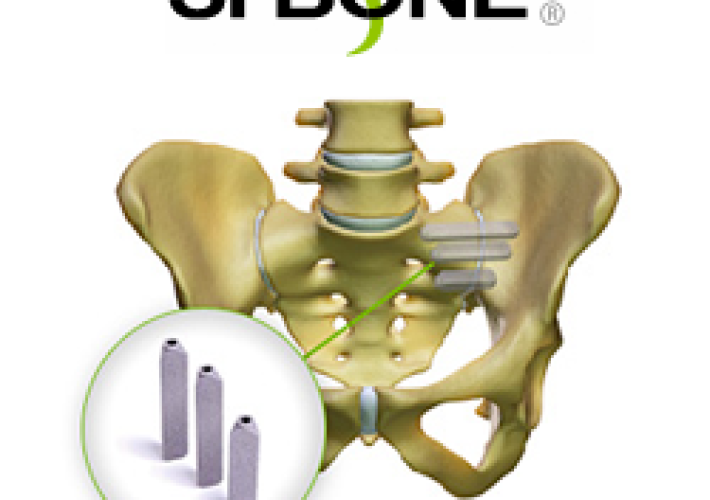
SAN JOSE, Calif., Aug. 9, 2017 /PRNewswire/ — SI-BONE, Inc., an innovative medical device company that pioneered the use of the iFuse Implant System® (iFuse), a triangular shaped minimally invasive surgical (MIS) device indicated for fusion for certain disorders of the sacroiliac (SI) joint, announced the publication of a systematic review of diagnostic accuracy studies titled Clinical classification in low […]

WARSAW, Ind., Aug. 8, 2017 /PRNewswire-iReach/ — Nextremity Solutions, Inc., a strategic commercialization organization with a focus on the musculoskeletal space, located in “The Orthopedic Capital of the World” Warsaw, IN, has announced the limited release of its newest product, the PiroVueTM Gastrocnemius Recession System for the treatment of equinus deformities in patients. According to a report in the […]

MARIETTA, Ga., Aug. 9, 2017 /PRNewswire/ — MiMedx Group, Inc. (NASDAQ: MDXG), the leading biopharmaceutical company developing and marketing regenerative and therapeutic biologics utilizing human placental tissue allografts with patent-protected processes for multiple sectors of healthcare, announced today that the Company has now distributed over 1,000,000 allografts to date for application in the Wound Care, Burn, […]

CAMBRIDGE, Mass.–(BUSINESS WIRE)–InVivo Therapeutics Holdings Corp. (NVIV) today provided a general business update and reported financial results for the quarter ended June 30, 2017. Mark Perrin, InVivo’s Chief Executive Officer and Chairman, said, “In the second quarter, we continued to make significant progress at InVivo. During the quarter, we enrolled four more patients into INSPIRE, and […]

MEMPHIS, Tenn.–(BUSINESS WIRE)–Active Implants, LLC, a company that develops orthopedic implant solutions, today announced enrollment of the 100th patient in clinical trials evaluating the NUsurface® Meniscus Implant for the treatment of persistent knee pain caused by injured or deteriorating meniscus tissue. The surgery was performed by orthopedic surgeon Dr. Wayne Gersoff of Advanced Orthopedics & Sports […]
LEEDS, United Kingdom–(BUSINESS WIRE)–Tissue Regenix Group plc, a UK- based regenerative medical company (AIM:TRX) announces completion of the acquisition of CellRight Technologies LLC, a US based specialist in regenerative osteoinductive bone scaffolds. The acquisition, expands the Group’s presence within the US healthcare market and increases US sales by more than double, bringing together two highly […]
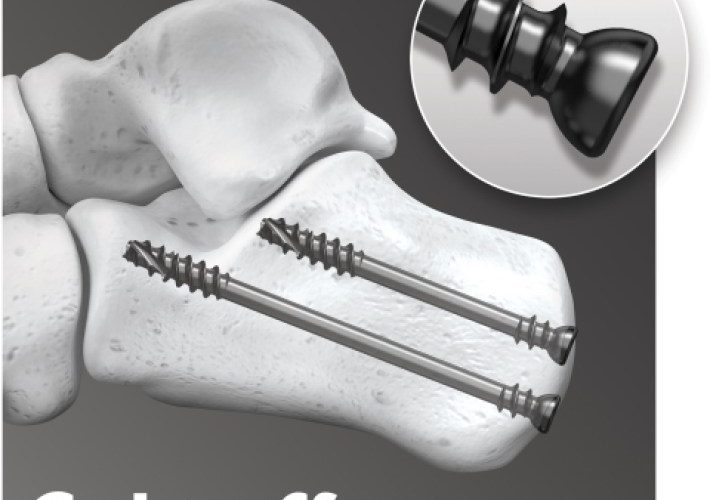
MEMPHIS, Tenn.–(BUSINESS WIRE)–In2Bones Global, Inc. today announced that its In2Bones USA, LLC subsidiary has received U.S. Food and Drug Administration (FDA) clearance for its new Fracture and Correction System. The System will be marketed under the 5MS™ Fracture Repair System and the CoLag™ Locking Compression Screw System brand names. The 5MS Fracture Repair System is a comprehensive plate and screw system […]